Apple Transforms AirPods into Assistive Listening Devices, Targeting the Lucrative Healthcare Sector
In the near future, individuals wearing AirPods might not just be tuning you out — they could be using them to enhance their hearing.
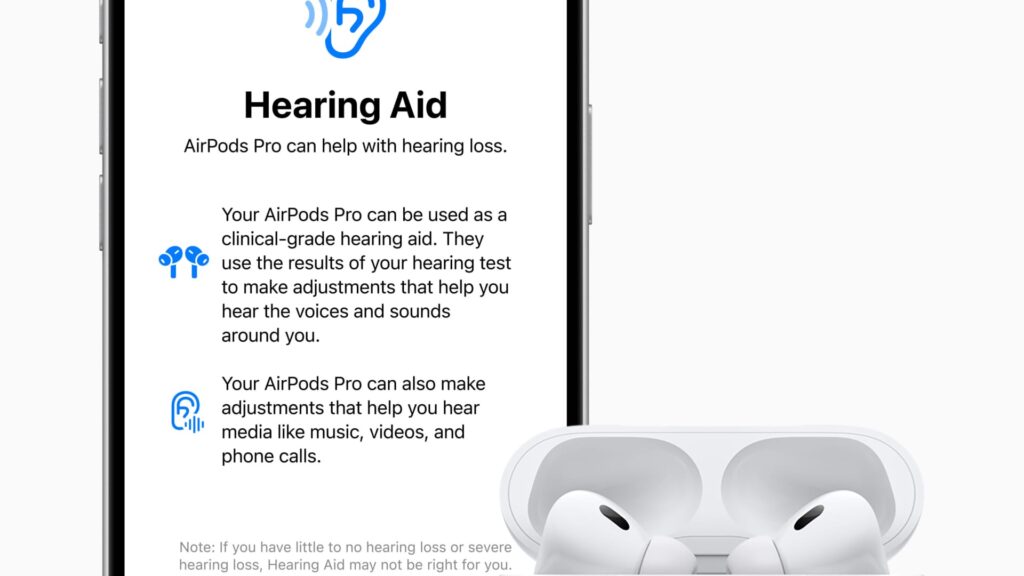
Apple revealed that its AirPods Pro 2 headphones will soon function as an FDA-approved hearing aid through a forthcoming software update. This development means that the roughly 30 million Americans with mild to moderate hearing loss can utilize Apple earbuds to amplify specific sounds they struggle to hear better.
“Once you’ve undergone a hearing assessment, your AirPods Pro will be converted into a personalized hearing aid, enhancing particular sounds you need in real-time, such as speech components or environmental elements,” stated Apple’s VP of health, Sumbul Desai, during the launch video.
The latest move underscores Apple’s initiative to enter the healthcare sector, a potentially $15 trillion market by 2030 as forecasted by RBC Capital Markets. Apple CEO Tim Cook has emphasized health-related functionalities as the company’s “most significant contribution to humanity.”
This strategy involves developing FDA-cleared features for their wearable products and substituting often pricier purpose-built medical devices. Since 2020, Apple has integrated notification services for irregular heartbeats, an atrial fibrillation reader, and an electrocardiogram reader into its Apple Watch, as per FDA submissions.
This novel feature will be a no-cost software update for select AirPods models and inclusive in Apple’s $249 AirPods Pro 2.
According to buyer guides referenced by the Hearing Loss Association of America, numerous over-the-counter hearing aids are notably more costly. While certain OTC hearing aids are priced as low as $99, the majority range from $799 to several thousand dollars.
Functionality Breakdown
The functionality of Apple’s hearing wellness experience necessitates both a pair of Apple’s AirPods Pro headphones and an iPhone.
Apple has incorporated a hearing assessment within its devices within the Settings application. Following the program’s confirmation that the headphones fit the user’s ears correctly, it plays a series of tones for approximately five minutes. The user must tap the screen when they detect a tone.
This process creates a profile of various frequencies and volume settings that may pose difficulty to the user, stored in the Health app. This profile can then be applied to tailor the AirPods Pro into personalized hearing aids.
Apple asserted that the assessment is scientifically sound, drawing upon data from its noise detection apps and a study involving 160,000 participants initiated in 2019.
In a promotional video, Apple depicted a mother utilizing AirPods to better perceive her son during her birthday.
Over-the-Counter Transition
Apple’s launch has received a boost from recent regulatory adjustments.
Previously, all hearing aids mandated a prescription post evaluation by a licensed audiologist. In 2022, the FDA expanded the market to encompass over-the-counter hearing aids that were notably more economical due to the utilization of audio testing software or at-home fittings.
However, Apple’s AirPods will not instantaneously render other hearing aids obsolete.
Among its constraints is the battery life, lasting six hours, inadequate for prolonged daily wear akin to some OTC hearing aids.
Furthermore, the AirPods Pro cater solely to individuals with mild to moderate hearing loss, encompassing those experiencing difficulty discerning speech in noisy environments. Individuals with severe or profound hearing loss still necessitate consultation with a licensed audiologist, as per experts.
Additionally, Apple’s hearing aids are still pending FDA clearance.
Devices leveraging technology or software to configure hearing aid fit or settings necessitate premarket clearance from the agency, as highlighted by an FDA press officer. Apple awaits FDA clearance alongside approval from regulatory bodies worldwide, as disclosed by the company on Monday.
Bridget Dobyan, executive director of the Hearing Industries Association, welcomed Apple’s foray into the market to enhance hearing health awareness. Nonetheless, various hearing loss scenarios warrant a physician-centered approach, as Dobyan pointed out.